Recent advancements in whole-body MRI have transformed our understanding of muscle disorders, particularly in diseases such as facioscapulohumeral muscular dystrophy (FSHD). FSHD, characterized by its heterogeneity in age of onset, rate of progression, and functional impact, presents significant challenges in clinical assessment and treatment. Historically, research focused on select muscle groups, neglecting the broader variability in muscle involvement across individuals, within muscles, and over the lifespan.
Innovations in MRI technology, such as high-resolution individual muscle analysis and within-muscle fat mapping, have illuminated previously underappreciated aspects of FSHD pathology, revealing patterns of fat infiltration and disease progression that were invisible using earlier imaging approaches.
These advancements underscore the potential of MRI-derived biomarkers to address longstanding challenges by offering reliable, scalable insights that reflect the disease’s whole-body impact and variability. This whitepaper will discuss challenges with existing biomarkers, explore how MRI addresses these gaps, and review recent data revealing new understandings of FSHD’s impacts and its characterization across a diverse population.
Functional Endpoints: Functional measures like the six-minute walk test and reachable workspace (RWS) assessments are valuable but often target specific muscle groups and can be influenced by learning effects or patient motivation, limiting their sensitivity for disease-wide changes.
Blood-Based Biomarkers: While systemic biomarkers, such as inflammatory cytokines or DUX4 expression, can provide insights into overall disease activity, they need to be paired with other measures like MRI to pinpoint specific muscle involvement or progression patterns.
Limited Sampling in Biopsies: Muscle biopsies provide direct DUX4 and histological data, but they fail to capture the spatial heterogeneity within affected muscles. Furthermore, biopsies are invasive, focus on a single muscle, and are often avoided by patients.
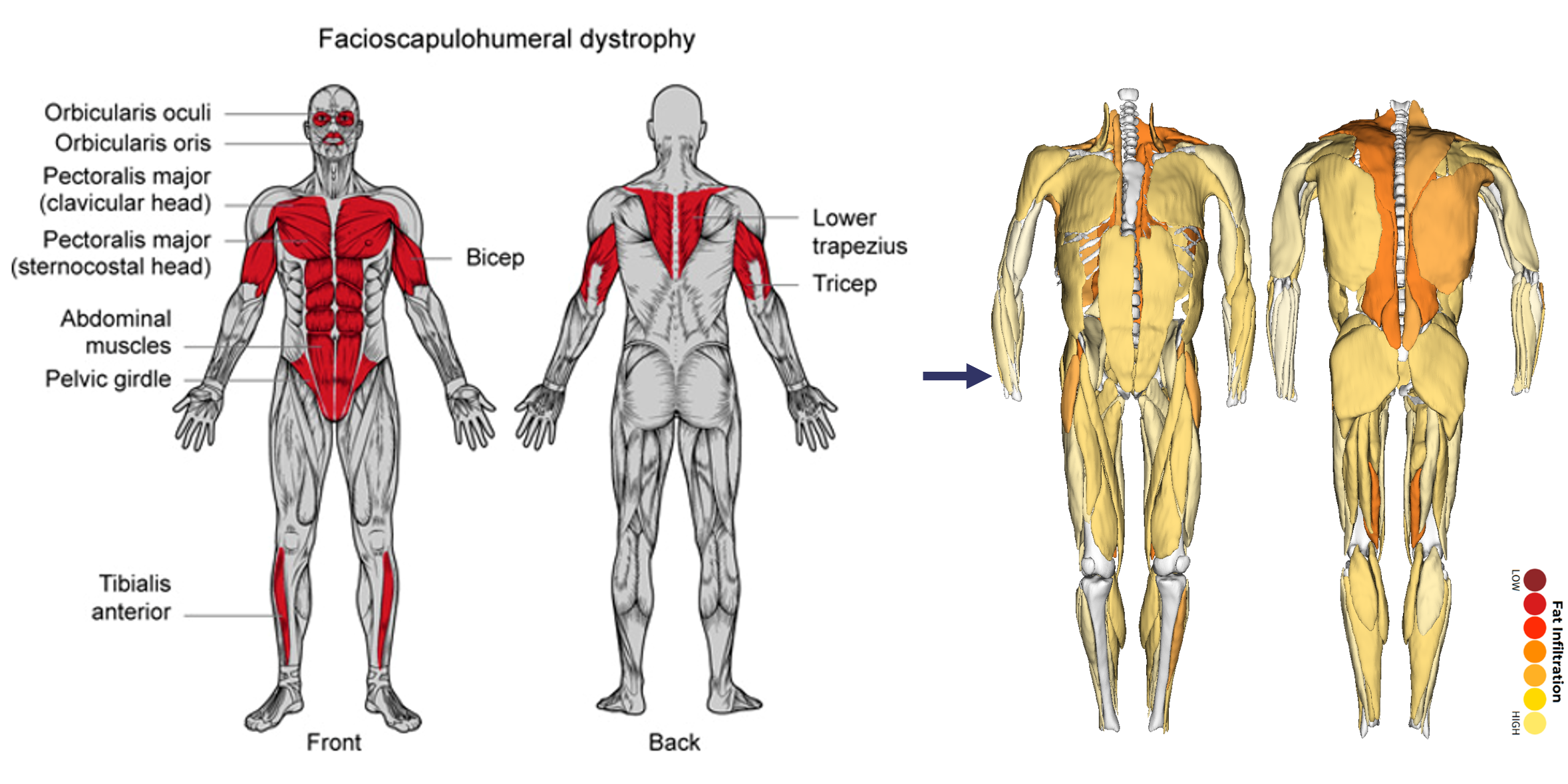
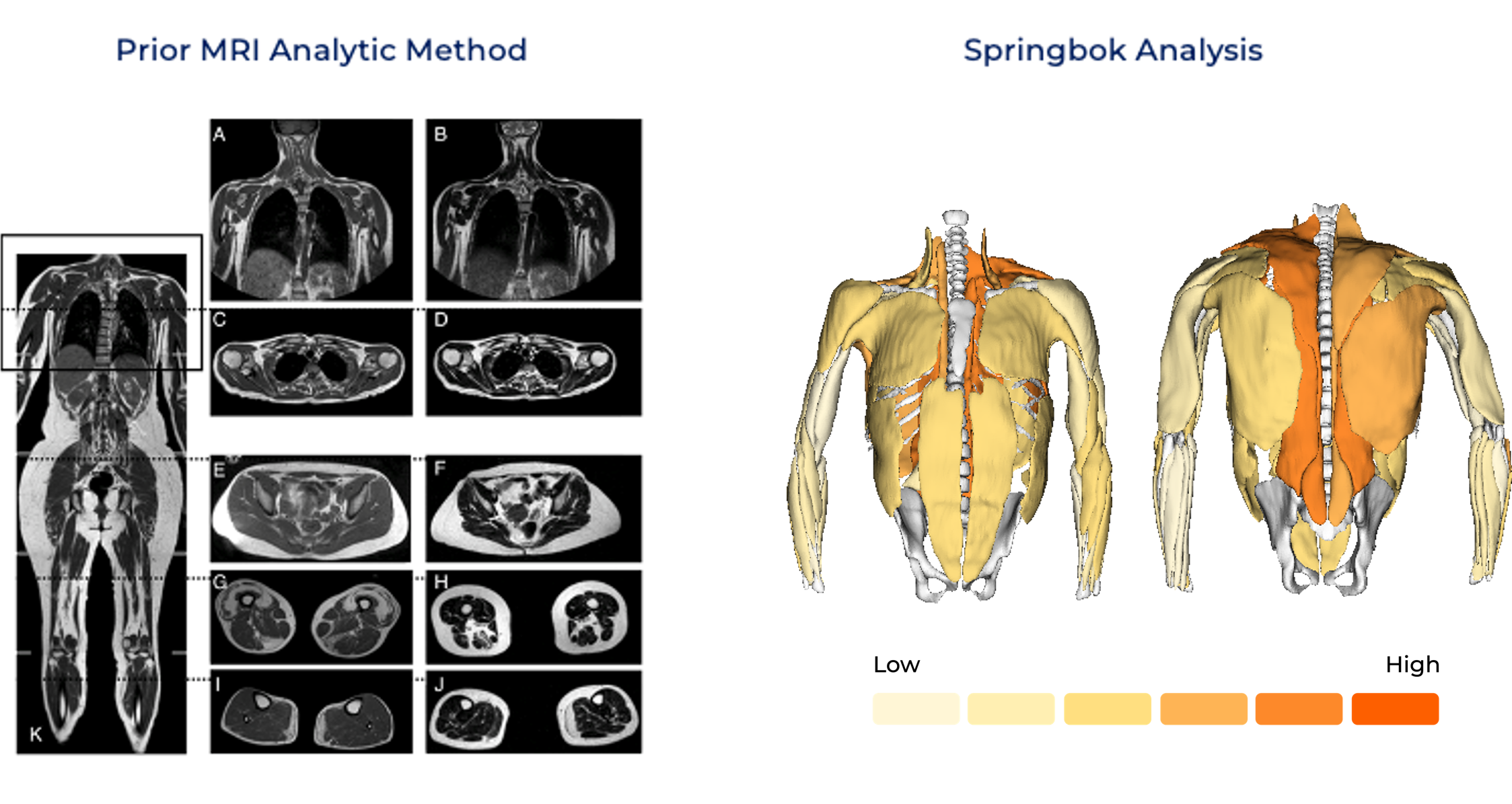
Underutilized MRI: Prior MRI analytic methods often relied on qualitative rating scales, muscle groupings, and/or limited slices of the thigh or calf regions. These lack the sensitivity to detect subtle or short-term changes in FSHD progression and overlook critical variations in disease progression within or between muscles. (See below)
AI-Driven MRI Analysis in FSHD
Automated AI-driven segmentation tools can quantify muscle volume, fat fraction, and inflammatory markers with unprecedented accuracy and efficiency.
These tools capture unique intramuscular patterns of disease that previously required labor-intensive manual segmentation or were left undetected. (See below)

Insights from Whole-Body MRI
Fat Infiltration Beyond Expected Patterns: Novel findings indicate fat infiltration does not always follow a simple distal-to-proximal gradient. U-shaped and other irregular patterns were observed, challenging conventional models of disease progression.
Muscle Involvement, Expected and Unexpected: Muscles Expected to Be Affected but Often Spared: Proximal muscles like the rectus femoris, typically implicated in muscle-wasting diseases, were observed to remain relatively unaffected in some patients, even at advanced stages, illustrating the variability in FSHD pathology.
Muscles Not Expected to Be Affected but Were: Some muscles showed significant atrophy or fat infiltration in certain patients, despite historically being overlooked. These changes correlated with functional deficits like impaired stability and gait.
Implication for Assessment: This heterogeneity underscores that focusing on a single muscle or muscle group is insufficient for capturing the disease's full impact. Whole-body imaging provides the necessary scope to identify both common and atypical disease manifestations, enabling more accurate tracking of progression and personalized therapeutic strategies.
Detecting Early Disease Changes
Early in the disease, when only a few muscles are affected, MRI can identify subtle changes in fat infiltration or inflammation before there is any corresponding functional impact. This makes MRI an indispensable tool for early detection and potential intervention.
Clinical Biomarker Development
MRI-based metrics of fat infiltration, volume asymmetry, and STIR demonstrate potential as sensitive, non-invasive biomarkers for monitoring FSHD progression and evaluating therapeutic responses.

Dynamic Visualization: Muscle-by-muscle MRI reveals disease dynamics at a scale and resolution impossible with prior technologies. Patterns of fat infiltration, muscle atrophy, and hypertrophy are mapped comprehensively, enabling precision targeting for interventions. (See below)
Data Aggregation and Scaling: The concept of an "MRI data lake" integrates datasets across multiple cohorts, allowing researchers to refine models of disease progression and build predictive analytics for therapeutic trials.
Implications for Clinical Trials: The enriched dataset provides a basis for adaptive clinical trial design, where biomarkers can be updated dynamically based on real-time patient data.
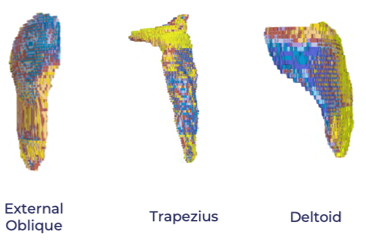
AI-driven whole-body MRI is revolutionizing how FSHD and other muscle conditions are understood and managed. By enabling detailed muscle-by-muscle analysis, these technologies allow for non-invasive, high-resolution insights that accelerate therapeutic innovation and improve outcomes for individuals living with FSHD.